The Properties Of Glass And How They Influence The Performance of Cleaning Solutions

Choosing the right cleaning solution for your windows starts with understanding the unique properties of glass. This knowledge allows you to select products that provide the best results. In this post, we will focus on float glass, the most commonly used type for windows, and explore how its characteristics influence the performance of various cleaning solutions.
What is Glass Made Of?
The main ingredient in glass is sand (which contains silica and lime) and soda ash:
- Silica (Silicon Dioxide): The main component of glass. Provides structural integrity and transparency.
- Calcium Oxide (Lime): Serves as a stabilizer, helping to prevent the glass from becoming soluble in water over time. It also enhances the glass's durability.
- Sodium Carbonate (Soda Ash): This additive lowers the melting point of silica, making it easier to work with during the manufacturing process. It also helps to increase the glass's resistance to water.
There are other additives that may be present in glass windows, including:
-
Magnesium Oxide: Sometimes added in small quantities, this helps to increase the glass's thermal resistance, making it suitable for use in windows.
-
Aluminum Oxide: This additive can enhance the glass's strength and scratch resistance, making it more durable and long-lasting.
How Is Glass Made?
While there are many types of glass, the most common type used in windows is float glass. It is produced by melting the ingredients (by heating the mixture between 2,600 and 2,800 degrees Fahrenheit) and floating the molten glass on top of a pool of molten tin. This results in a long, continuous ribbon of glass with a smooth and uniform surface. The glass is gradually cooled as it travels down the line until it is hard enough to be cut into smaller pieces.
Cleaning Solution Requirements For Glass Properties
Non-Porous And Smooth Surface
-
The smooth, non-porous surface of glass means that dirt and contaminants sit on the surface rather than being absorbed.
-
Cleaning Solution Requirement: Solutions must be able to loosen and remove these surface contaminants. Surfactants are crucial for this, because they reduce surface tension, allowing the cleaning solution to spread evenly and penetrate under dirt particles.
Microscopic Imperfections
-
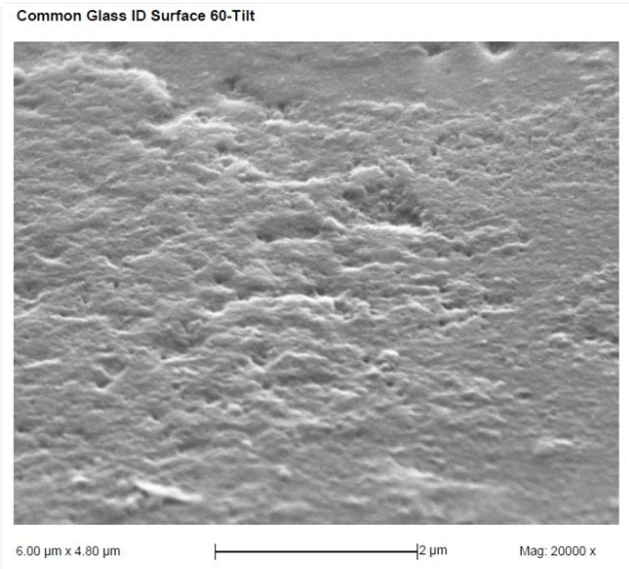
When float glass is lifted from the molten tin bath, traces of tin or tin oxide are deposited on its surface, creating what is known as the “tin side." The opposite surface, exposed to air, is referred to as the “air side.” On a microscopic level, the tin side looks smooth, while the air side looks rough, with pits and hills. (see image below)
-
Cleaning Solution Requirement: When surfactants in soap mix with calcium in hard water, they form a soapy, slimy scum instead of foam. To deal with this, a foaming agent is added to create artificial foam, giving the illusion of effective cleaning. However, this mixture, combined with the soap scum and calcium, forms a sludge that clogs microscopic pores on glass surfaces. Using a cleaning solution without these additives, such as Glass Gleam from Titan Labs, avoids the sludge and clears the pores, allowing light to pass through fully.
Transparency And Reflectivity
-
Any residue or streak left on glass is highly visible due to its transparency and reflective properties.
-
Cleaning Solution Requirement: The cleaning solution must evaporate quickly and completely to avoid leaving streaks. Ingredients like isopropyl alcohol and ammonia are often included because they evaporate rapidly, minimizing residue.
Chemical Reactions With Some Acids
-
Not many acids can dissolve glass, but those containing fluorine can. The most common being hydrofluoric acid (HF). HF reacts with the silica in glass, actually breaking its chemical bond. It can also react with the tin on the “tin side” of the glass, causing the glass to become hazy. Hydrofluoric acid is actually used in the fabrication process to create frosted glass.
-
Cleaning Solution Requirement: Products containing hydrofluoric acid, such as Winsol Crystal Clear 550, can work great at removing hard water stains, but they also have the potential to “smoke” or etch the glass. It's best to start with the most mild acids before trying a product with hydrofluoric acid in it. Also, testing on a small area should be done when using any solution containing acids.
Understanding the properties of glass is important for selecting the right cleaning solutions. By considering its non-porous nature, microscopic imperfections, and transparency, you can choose products that clean effectively without residue or damage.
Leave a comment